Calculate Absorbance using Beers Law. Alternatively for samples which scatter light absorbance may be defined as the negative logarithm of one minus absorptance as measured on a uniform sample.

1 2 Beer S Law Chemistry Libretexts

Linear Equations Represent The Linear Absorbance Response To Dose For Download Scientific Diagram

Linear Equation Curve Between Inhibition Absorbance Versus Download Scientific Diagram
Given the form of eqs.

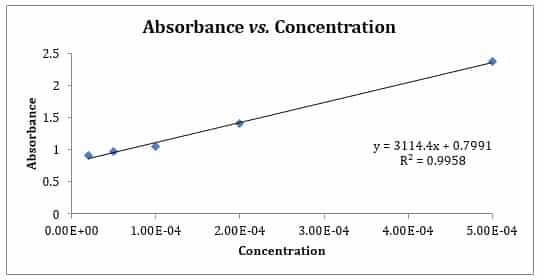
Linear equation for absorbance. Absorbance is defined by the equation A -logT or A logloI. The thick straight line in Figure 1 is the linear regression that best describes the entire set of standard points R 2 09355. Optical Density for Absorbance Measurements October 22 2012 For absorbance measurements the optical density OD is a logarithmic measurement of the percent transmission T and it can be represented by the equation A log10 100 T.
Concentration and absorbance is best described by a straight line. For y 06 x 854µgmL. The equation for this line is y 000056x 01174.
Also the value R2 0987 for this line was. This indicates that the measured absorbance values are highly similar to the expected values generated by the linear line. Where A is the absorbance.
As shown in the figure the equation of the approximated line is best described as. Solving for x gives x 17704y -20791. This translates to concentrations between 100 nguL and 3700 nguL when using the Nanodrop ND1000.
It is the linear relationship between absorbance and concentration of an absorbing species. The concentration of one or more solutions containing unknown amounts of Allura Red will be determined experimentally by the use of the trendline equation. For example the equation for the heart rate-speed experiment is rate633573749speed.
According to the Beer Lambert Law the Absorbance is proportional to the path length distance that light travels through the material and the concentration of the material. Absorbance is defined as the logarithm of the ratio of incident to transmitted radiant power through a sample excluding the effects on cell walls. What is the significance of the molar absorptivity e.
7 and 8 this result is surprising since it is not clear how integrating the absorption index or the absorbance results in a linear relation between the integrated absorbance and concentration. Beers Law AEbc helped to develop the linear equation since absorbance was equal to y Eb was equal to m and the concentration c was equal to the slope x in the equation ymxb. Concentration 71758 x Absorbance 4 The line has a slope of 71758 and an R2 value of 09779 from a linear fit.
Where a is the Absorbance e is the Molar absorption coefficient l is the Path Length and c is the Concentration. Once you know a and b you can use this equation to predict the value of Y for a given value of X. The linear equation shown on the chart represents the relationship between Concentration x and Absorbance y for the compound in solution.
Some instruments can measure up to 20 unit absorbance and you need to examine whether your sample measurement is still linear in this area. The linear relationship between absorbance and concentration displays that absorbance depends on the concentration. Enzyme kinetics is the study of the rates of enzyme-catalysed chemical reactionsIn enzyme kinetics the reaction rate is measured and the effects of varying the conditions of the reaction are investigated.
The equation for the best line is. Samples outside of this range should be drieddown or diluted to produce more accurate spectrophotometry results. The term is used in many technical areas to quantify the results of an experimental.
Rydberg formula predicts the wavelength of light in the vacuum that is emitted during a electronic transition between different energy levels for Hydrogen or. Thermal expansion is normally calculated in two ways as a linear single dimension or volumetric 3 dimensional measurement. Additionally the other piece of information that is required for any standard curve is the equation of the line.
L is the path length the distance of solution that the light has to travel through. Where k is calculated from Equation 7 in combination with Equation 9. The linear equation was absorbance 00189 x 000566.
What is the quantity of chemical B in a sample that gives a measured absorbance of 0189 when a blank has an absorbance of 00789. The proportionality constant of the equation is termed as the molar extinction coefficient of the substance. When plotted on a graph of Absorbance units against Time the linear region usually corresponding to the initial rate of the resulting graph will give you the velocity of the reaction in Absorbance units per second.
A plot of absorbance against concentration for Allura Red will be constructed. Unlike the absorbance at a single wavelength of 590 nm the ratio of absorbance values 590 nm over 450 nm is linear with protein concentration Fig. Standard addition was used to determine Cu in a 100 L water sample from Lake Jasper.
Add the zero timepoint using your value from the prelab questions and your equation from 2. To begin we will rearrange the equation A e bc. This allows you to convert between absorbance and mg of starch.
The linear relationship between concentration and absorbance is both simple and straightforward which is why we prefer to express the Beer-Lambert law using absorbance as a measure of the absorption rather than T. While working in concentration units of molarity the Beers law is written as aecl. Depending on the case each method is useful as finding out how a beam would extend due to heat would only require a linear method however when trying to find out how a body of liquid expands with temperature volumetric might be best.
A linear trendline will be added to the plot. ε is the molar absorptivity constant. A straight line was approximated through the data points using linear regression.
Repeat this for each concentration of catechol but. The main absorbance equation is the Beer-Lambert Law which is. C is the concentration of the solution.
Measured Absorbance by Spectrophotometric. Frequently the absorbance A rather than transmittance is used for the amount of light a solution absorbs. The protein concentration of the unknown sample may be simply calculated using the linear equation of the calibration curve Fig.
Print the polished graph and staple it in your notebook. The regression line can be considered an acceptable estimation of the true relationship between concentration and absorbance. BeerLambert equation is only linear for absorbances between 01 and 10.
The equation for the regression line is usually expressed as YabX where a is the Y intercept and b is the slope. Studying an enzymes kinetics in this way can reveal the catalytic mechanism of this enzyme its role in metabolism how its activity is controlled and how a drug or a modifier inhibitor. Summarize your absorbance data for the amylase assays in well labeled tables.
The absorbance of a solution depends on the quantity of light absorbing species in the solution and the length of the solution the light passes through. Alternatively the results can be interpreted as 9996 of the variance being explained by the linear line. The R2 value for the best fit line was 0987.
Therefore some method can be. This is different for every chemical and at every wavelength. Include the equation of the line on the plot.
The second hypothesis that a linear relationship between absorbance and concentration would be found was also not falsified based on the experiment. Y 20476X 00211.

Equilibrium Constant Turn In Cv Final Report And Pre Lab In Folders Ppt Download

Beer Lambert Law Transmittance Absorbance Edinburgh Instruments
Lab 2 Beer S Law And Molar Extinction Coefficients Colorimeter User Manual

Simulation Of Instrumental Deviation From Beer S Law
Beer S Law Lab Explained Absorbance Vs Concentration Schoolworkhelper

A Linear Equation Of Biosensor With Absorbance Value 0 500 Between Download Scientific Diagram
Untitled Document
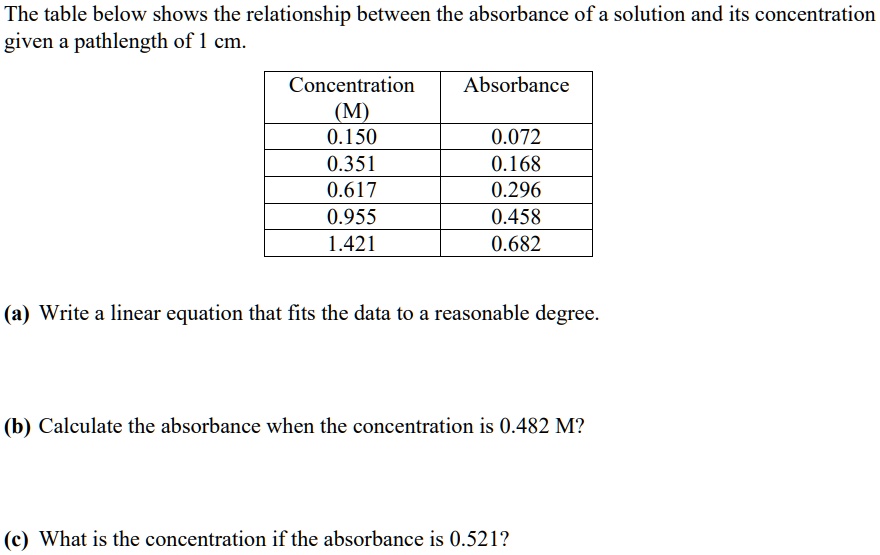
Solved The Table Below Shows The Relationship Between The Absorbance Of A Solution And Its Concentration Given Pathlength Of 1 Cm Concentration Absorbance 0 150 0 351 0 617 0 955 1 421 0 072 0 168 0 296 0 458 0 682
- Pelan Tindakan Kbat
- Borang Guru Ganti Ppd Seberang Perai Utara
- Amazon Cloud Drive Plans
- Menurut Model Akibat Logikal Dreikurs
- Cara Konkrit Lantai Yang Betul
- This Or That Questions For Couples
- Subject For Diploma Akaun
- Pentadbiran Berjaya Di Sabah
- Fomema Johor Bahru
- Cara Cara Menyimpan Duit Dengan Betul
- Format Akaun Semasa Prinsip Perakaunan
- Sistem Pentadbiran Di Negeri Perak Sebelum Kedatangan Kuasa British
- App Programmeren Ios
- Program Advokasi Pencegahan Kemalangan
- 16 Digit Nombor Akaun Bank Mana
- Citadel Hedge Fund
- Layakkah Graduan Universiti Malaysia Bekerja Di Luar Negara
- Tiger Woods Kids
- Six Days In Fallujah
- Portal Rasmi Pentadbiran Tanah Johor
