Eventually the vibrating molecules release the radiation which will likely be absorbed by another greenhouse gas molecule. Atmospheric methane is the methane present in Earths atmosphere.

3a Co2 It S A Gas

The Earth S Radiation Budget Science Mission Directorate
New Page 2
Most incoming shortwave radiation from the Sun passes through the atmosphere without being absorbed.

Why carbon absorb radiation. In making a case against CO 2 as a greenhouse gas. But carbon dioxide and other trace gases in the atmosphere do. They have also discovered that the sansevieria plant is an anti-pollutant and can on occasion be used as an antidote to certain forms of radiation.
Then compare to the effect of glass panes. Consisting of a single carbon atom joined to four hydrogen atoms CH 4 like CO 2 is found in minute quantities in the atmosphere but it can have a sizable impact on global warming. Plankton absorbs Carbon-14 from the ocean much like terrestrial plants absorb Carbon-14 from the air.
Atmospheric methane concentrations are of interest because it is one of the most potent greenhouse gases in Earths atmosphere. Oxygen and Nitrogen in the air mostly ignore it but Carbon Dioxide molecules have the geometry and composition that allows them to absorb the radiation of this wavelength. Trees are part of the natural carbon cycle so growing them doesnt meaningfully reduce CO2 levels and burning or decaying trees dont meaningfully increase CO2 levels from what the level of CO2 would be due to natural processes.
Oxygen and nitrogen with just two atoms in their molecules do not absorb infrared radiation. This process keeps heat near the Earths surface. The more carbon dioxide that people pumped into the atmosphere by burning fossil fuels the more the oceans would absorb.
Methane is a powerful greenhouse gas with a capacity to absorb considerably more heat molecule for molecule than carbon dioxide can. Was the first to quantify the contribution of carbon dioxide to what scientists now. In turn the warmed atmosphere emits longwave radiation some of which radiates toward the Earths surface keeping our planet warm and generally comfortable.
But Earth stays warm even at night because of a layer of carbon dioxide or CO 2 in our atmosphereCO 2 acts like a heat-trapping blanket absorbing the heat and holding it in. Methane CH 4. That is over a 20-year period it traps 84 times more heat per mass unit than carbon dioxide CO 2 and 105.
The Sun emits radiation from X-rays to radio waves but the irradiance of solar radiation peaks in the visible wavelengths see figure below. A loose necktie on Wikimedia Commons When sunlight reaches Earth the surface absorbs some of the lights energy and reradiates it as infrared waves which we feel as heat. Greenhouse gases in the atmosphere such as water vapor and carbon dioxide absorb most of the Earths emitted longwave infrared radiation which heats the lower atmosphere.
Plants are not the only organism that can process Carbon-14 from the air. Carbon dioxide and other heat-trapping gases have three or more atoms and frequencies that correspond to infrared radiation emitted by Earth. These units are the units of spectral irradiance which is also.
Usually this free oxygen atom quickly re-joins with an oxygen molecule to form another ozone molecule. However the increasing atmospheric CO2 concentrations driven by human-caused emissions are forcing the ocean to now absorb this gas. The main sources of carbon include the combustion of fossil fuels coal oil and gas for the sake of energy and transportation by humans fires also includes wildfires and farmland.
Common units of irradiance are Joules per second per m 2 of surface that is illuminated per nm of wavelength eg between 300 nm and 301 nm or W m 2 nm 1 for the plot below. A Heat-Trapping Blanket Sunlight warms the surface of the Earth. Zoom in and see how light interacts with molecules.
Because of this ozone-oxygen cycle harmful ultraviolet radiation is continuously converted into heat. There are other heat-trapping gases in the atmosphere but CO 2 is the most important one These include methane CH 4 water vapor nitrous oxide. Ozone can absorb wavelengths between 9 μm and 10 μm but as you have learned it is found in low concentrations.
Deforestation is the second largest anthropogenic source of carbon dioxide to the atmosphere ranging between 6 percent and 17 percent. Most of the gas in the atmosphere is nitrogen and oxygen which cannot absorb heat and contribute to the greenhouse effect. Scientists have found that the Sanseveira can harmlessly absorb over 100 different types of poison.
Do all atmospheric gases contribute to the greenhouse effect. Change the greenhouse gas concentration and see how the temperature changes. Over the last century the burning of fossil fuels like coal and oil has increased the concentration of atmospheric carbon dioxide CO 2This happens because the coal or oil burning process combines carbon with oxygen in the air to make CO 2To a lesser extent the clearing of land for agriculture industry and other human.
How do greenhouse gases affect the climate. They absorb more carbon than they release whereas carbon source is anything that releases more carbon than they absorb. Since plankton is the foundation of the marine food chain Carbon-14 is spread throughout aquatic life.
Diffuse sky radiation is solar radiation reaching the Earths surface after having been scattered from the direct solar beam by molecules or particulates in the atmosphereAlso called sky radiation the determinative process for changing the colors of the skyApproximately 23 of direct incident radiation of total sunlight is removed from the direct solar beam by scattering into the atmosphere. The suns ultraviolet wavelengths are strongly absorbed by ozone in the stratosphere. Trees and vegetation absorb carbon when they grow but then release it as carbon dioxide when they die and decompose.
The 20-year global warming potential of methane is 84. While the ability of the ocean to capture and store carbon has helped to slow the accumulation of atmospheric CO2 and hence the pace of global warming it has come at a cost. Water vapor and carbon dioxide can absorb radiation wavelengths in the range of 4 μm to 80 μm except those between 8 μm and 12 μm.
Gas molecules that absorb thermal infrared radiation. Greenhouse gases like carbon dioxide and methane absorb the infrared energy re-emitting some of it back toward Earth and some of it out into space. On Earth human activities are changing the natural greenhouse.
We hear a lot about carbon dioxide when we talk about climate change but sometimes its important to go back and examine why too much CO2 in the atmosphere is a bad thing. Why Carbon Dioxide Is a Greenhouse Gas. The ocean would continue to soak up more and more carbon dioxide until global warming heated the ocean enough to slow down ocean circulation.
They get excited and re-radiate the energy again as infrared back out. This website provides an interactive guide to the issues of global warming and climate change targeted to a middle school audience but accessible to the public at large. Explore the atmosphere during the ice age and today.
Atmospheric methane is rising. When an ozone molecule absorbs even low-energy ultraviolet radiation it splits into an ordinary oxygen molecule and a free oxygen atom. What happens when you add clouds.
Greenhouse gases are atmospheric gases that absorb infrared radiation and trap heat in the atmosphere. The idea seemed simple enough. Carbon sinks can be natural or man-made.
Why Does Carbon Dioxide Absorb Infrared Radiation Quora

John Tyndall
Greenhouse Effect
Global Warming Potential Carbon Offset Guide
The Greenhouse Effect
Why Does Carbon Dioxide Absorb Infrared Radiation Quora
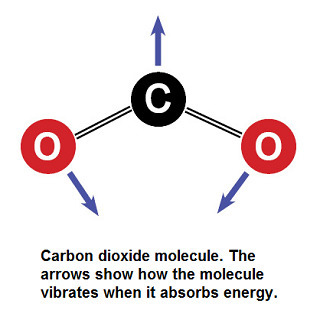
Carbon Dioxide How Can A Little Co2 Molecule Be Such A Big Troublemaker
Greenhouse Gases
