L is the pathlength if you use cuvette of 1 cm then you can calculate c. The Beer-Lambert law or Beers law is the linear relationship between absorbance and concentration of an absorbing species.

What Factors Will Affect Absorbance Ecuvettes
Absorbance Theory Amp Application Turner Designs

Relation Of Cell Path Length Vs Absorbance Of Qusomes Gdm 12 In Download Scientific Diagram
The optimal protein concentration is a function of the pathlength of the cuvette.

Pathlength and absorbance. Someone mentioned microplate readers. As shown in Figure 1 absorbance and concentration were directly proportional to each other. The Molar Absorptivity Constant is specific for every single solution and at every wavelength.
Higher absorbance coefficients indicate that lower amounts of incident light will pass through the material. Absorbance taken for 0 to 60 minute rate of 1 min for total 61 readings. Absorbance measurements at 1 cm pathlength have been correlated with specific nucleic acid concentrations.
For example an OD of 10 at 260 nm correlates to 50 μgml of dsDNA Table 1. Unlike optical absorbance the absorbance coefficient represents the optical attenuation per unit length of material typical units are 1cm. Exploring absorbance-based assay applications.
The absorbance of a material that has only one attenuating species also depends on the pathlength and the concentration of the species according to the BeerLambert law where ε is the molar attenuation coefficient of that material. 8 samples in 20 sec. This is a measure of how easily light passes through a material.
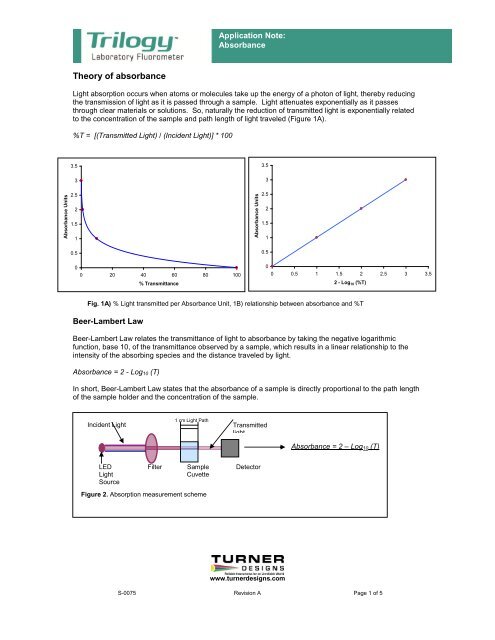
A Log 10 I 0 I. When you are taking an absorbance spectrum and measuring the absorbance at different wavelengths this is the only factor that is changing as the concentration of the solution remains the same and so does the pathlength. However the addition of the second drop to the first cell has exactly the same effect as adding it.
Since the cell pathlength for this. Microplate readers with absorbance read capability are widely used in biological and. The liquid pathlength of each well is then calculated and absorbance values are corrected accordingly corresponding to the 1 cm pathlength as shown in equation 5.
The absorbance is related to the concentration of the molecules absorbing the light by Beers Law. When you are taking an absorbance spectrum and measuring the absorbance at different wavelengths this is the only factor that is changing as the concentration of the. A ε b C C is the molar concentration of the absorbing molecules.
Determine concentration using the Beer-Lambert Law. Dimensions L x W x H 24 cm x 33 cm x 17 cm 94 in x 13 in x 67 in Lamp. While the NanoDrop Lite is designed with fewer features than the NanoDrop 2000 or NanoDrop 8000 seri.
003 - 145 mgmL IgG. ℓ is the pathlength. The general Beer-Lambert law is usually written as.
The DS-11 can measure samples with absorbance values as high as 750 absorbance units at a 1 cm equivalent path length. The Thermo Scientific NanoDrop Lite Spectrophotometer is a compact personal UV-Vis microvolume spectrophotometer that complements the full-featured NanoDrop 20002000c and NanoDrop 8000 instruments. Where I 0 is the intensity of the incident light and I is intensity of that light after it passed through the sample.
One of the most used methods for determining nucleic acid concentrations is based on measuring the optical density of a sample at 260 nm OD260. This translates into 1125 mgml of BSA protein or 37500 nguL of dsDNA. Through the relationship between absorbance A and three variables.
Figure 4 shows a plot of the protein concentration required to produce an absorbance of 05 2. Different disciplines have different conventions as to whether. B is the cell pathlength which is the distance a photon.
Buy spectrometer accessories in the mid and far infrared such as ATR Specular Reflectance Diffuse Reflectance and Transmission FTIR. The section curve is then analyzed in real time to verify linearity in compliance with the Beer-Lambert law. Optical pathlength correction for absorbance measurements.
Between sample absorbance and signal to noise ratio is illustrated in Figure 31. NanoDrop Eight Spectrophotometer with Laptop USCAN. Therefore the concentration of Allura Red can be determined by measuring the absorption of light through the solution.
From virus to cannabis research. This is lower than the optimal 09 to account for absorbing buffer components. Absorbance also known as optical density is the quantity of light absorbed by a solution.
In this case C 400 x 10-6 moleL. A a b c where A is the measured absorbance a is a wavelength-dependent absorptivity coefficient b is the path length and c is the analyte concentration. With advances in instrumentation you can get absorbance of 2 and greater.
So the absorbance is also a function of pathlength L. Any molecule with absorbance at 280 nm will contribute to the total absorbance used to calculate sample concentrations. When the concentration is.
The addition of a drop to the second cell reduces the transmittance to 49Abs 0310 doubling the absorbance as is expected by Lamberts Law since the pathlength of colored material is doubled. By varying the pathlength with a precision linear stage Slope Spectroscopy eliminates the need to manipulate the sample and decreases errors. 20 - 10000 ngμL dsDNA.
Triton X-100 and NP-9 are two examples of components found in common buffers that may contribute to the total absorbance values at 280 nm. According to the Lambert-Beer law. Guide to understanding and working with the BeerLambert law According to the BeerLambert law the concentration of a protein is directly proportional to its absorbance at a.
This ultra high absorbance capability is achieved by using a remarkably small pathlength enabled by our SmartPath Technology. T II 0 and T 100 T. When using a 1 cm cuvette the pathlength is 1 and equation.
Unlike the single value dependence of legacy UV-Vis methods the data-dense slope method characterizes samples by collecting multiple absorbance data points at several pathlengths to create a section curve absorbance vs. The Molar Absorptivity Constant is specific for every single solution and at every wavelength. C is the molar concentration of those species.
004 200 Abs 10 mm equivalent Description. Enzyme amount was constant. A 2 - log 10 T.
Leveraging the power and flexibility of variable pathlength technology Slope Spectroscopy is a rapid and robust concentration measurement method. Where A is the absorbance unitless is the molar absorptivity coefficient M-1cm-1 l is the pathlength of the light through the cuvette cm and c is the concentration M. The equation that allows one to calculate absorbance from transmittance is.
A εbc Beers Law The three variables are concentration of the solution c the pathlength of the light through the solution b and the sensitivity of the absorbing species to the energy of the analytical wavelength. Based on Beers Law A εcl4 the slope of the line should be equal to the molar absorptivity multiplied by the cell pathlength.

Uv Vis Absorbance Spectrum Of Sucrose Solutions Over 10 Open I

Absorbance Per Unit Path Length L 660 Nm A L As A Function Of Download Scientific Diagram

What Is Pathlength And Why Is It Important When Selecting A Sample Interface

What Is Absorbance Absorbance Measurement Absorbance Assays Molecular Devices
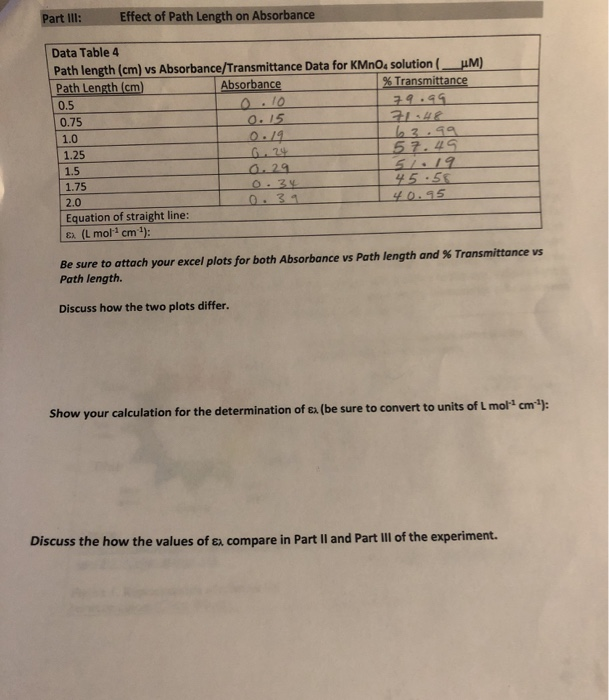
Solved Part Iii Effect Of Path Length On Absorbance Data Chegg Com
Beer S Law Theoretical Principles

Variable Pathlength Cell Wikiwand

Our Technology Ctech Analytical Solutions
