A molecular picture of heat conduction will help justify the equation that describes it. THERMA V LG THERMA V is an Air to Water Heat Pump AWHP System that offers heating and hot water solution.
Chapter 5 Thermochemistry

Thermochemistry Chapter 6 Semester 6 1 The Narure Of Energy And Types Of Energy 6 2 Energy Changes In Chemical Recations 6 3 Introduction To Thermodynamics Ppt Download
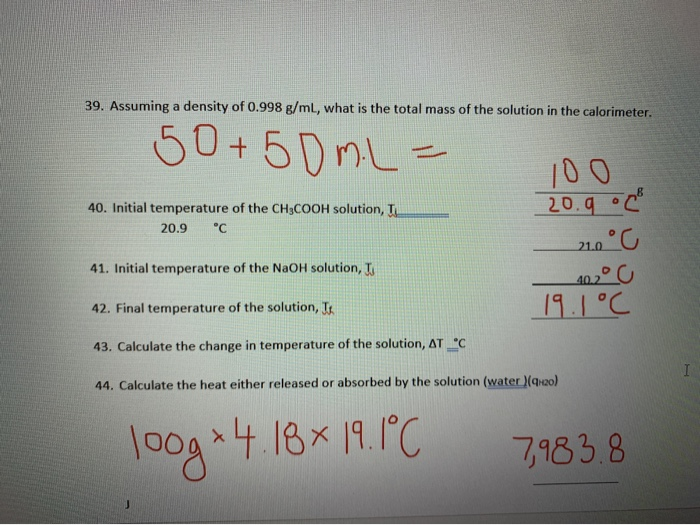
The Heat Absorbed By Solution Solo Is Then Equal To Chegg Com
Heat energy can also be absorbed by the breaking and formation of bonds between molecules.
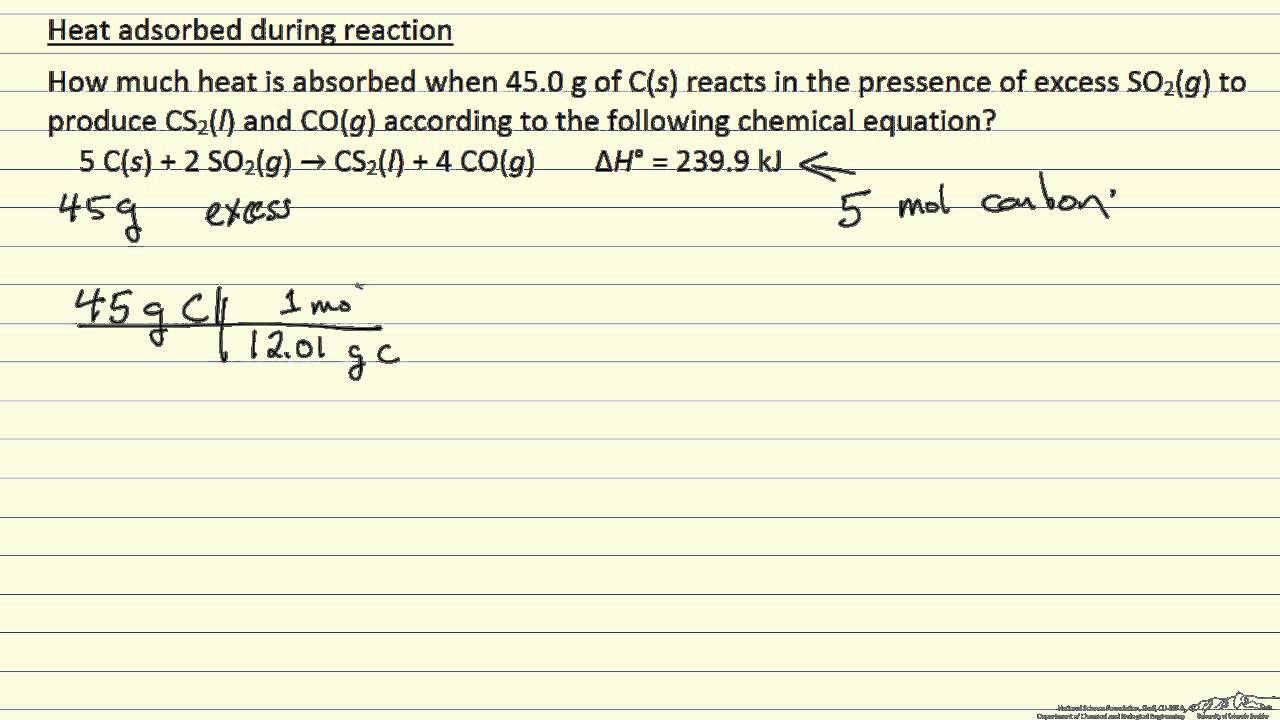
How to calculate heat absorbed by solution. This is a different process from adsorption since molecules undergoing absorption are taken up by the volume not by the surface as in the case for adsorptionA more general term is sorption which covers absorption adsorption and. We will first calculate the heat q 1. Heat released by 5 kg of steam at 100C to convert into water at 100C.
Specific latent heat of vaporization of steam is 2268 kjkg Solution. The sensible heat formula is used to calculate the flow of air in the electric furnace. The temperature of the water rose to 275C.
The total amount of heat depends upon the number of molecules dictated by the mass of the object. Calculate the change in enthalpy ΔH for these two processes. ML 5 2268 KJ 11440 kj.
Heat is a measure of molecular energy. Calculate the amount of heat absorbed when 688 grams of ice at -300 degrees Celsius is converted to steam at 160 degrees Celsius. When heat is absorbed by the solution q for the solution has a positive value.
Temperature on the other hand measures the average energy of each molecule. The heat capacity of the calorimeter must be determined experimentally. Calculate the mass of butan-l-ol needed to burn completely in excess oxygen in order to raise the temperature of 500 cm 3 of water by 35C.
One BTU is equal to the amount of heat required to raise the temperature of one pound of water one degree Fahrenheit. Density equals 100 gmL and specific heat equals 4184 J g 1 C. How to Calculate Heat Absorbed by the Solution.
The heat that is either absorbed or released is measured in joules. The easiest process is to study the mixing of warm and cold water. Q m c g T final - T initial q m c g ΔT.
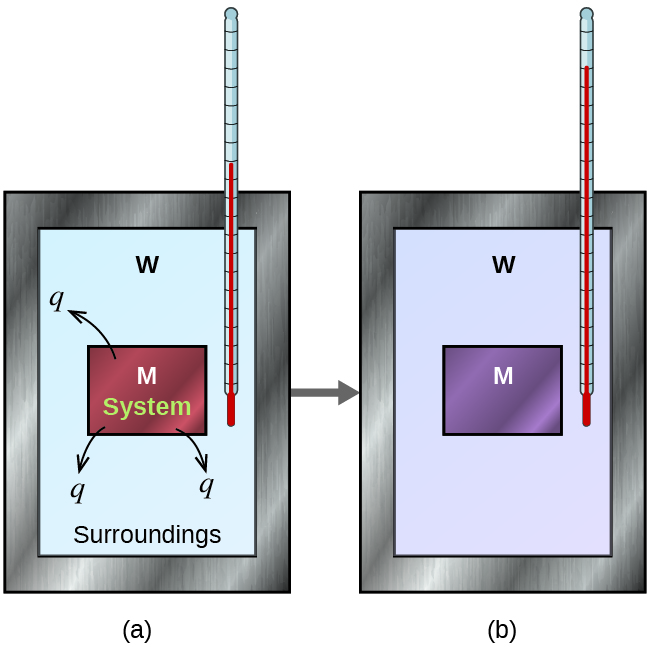
In a certain experiment 500 g of NaOH is completely dissolved in 1000 L of 200C water in a foam cup calorimeter. When an endothermic reaction occurs the heat required is absorbed from the thermal energy of the solution which decreases its temperature Figure 1. The molar heat of solution of NaOH is -4451 kJmol.
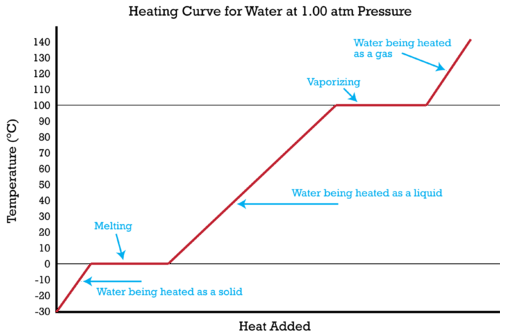
The amount of heat released or absorbed per gram or mole of reactant can then be calculated from the mass of the reactants. Calculate the heat released or absorbed in joules when the solute dissolves in the solvent. The phase shift between the solid liquid and gas is relevant to the latent heat.
Specific heat capacity of water. To determine the amount of heat energy absorbed by a solution you must do more than find its temperature. In a metal the picture would also include free valence electrons colliding with each other and with atoms likewise.
Heat is frequently measured in British Thermal Units BTU. We can calculate the amount of heat absorbed by the solution or the amount of heat removed from the solution with the following equation. What is the direction of heat flow.
Assuming no heat loss calculate the final temperature of the water. Calculate the enthalpy change heat of solution for the reaction in kJ mol-1 of solute. In chemistry absorption is a physical or chemical phenomenon or a process in which atoms molecules or ions enter some bulk phase liquid or solid material.
The LG Therma V Air to Water Heat Pump uses renewable technologies and natural energy from the air to provide green heating and hot water for your home. Calculate the joules of heat absorbed or released during a process using the mass of the substance its specific heat capacity and the change in temperature during the process. To find the heat absorbed by the solution you can use the equation.
Heat released or absorbed mass specific heat capacity change in temperature. Complete combustion of 1 mole of butan-l-ol C 4 H 9 OH produces 2678 kJ of heat. If the specific heat capacity of the solution is 4184 JCg and the heat capacity of the Styrofoam cup is negligible calculate the molar heat of solution of PCl 3 in water.
Q sensible 110 x c fm x t o. Also assume that the density and specific heat of the dilute aqueous 100 M HCl solution are the same as that of pure water. The idea here is that you can use the heat absorbed by the solution to find the heat given off by the dissolution of the salt.
The specific heat of a substance can be used to calculate the temperature change that a given substance will undergo when it is either heated or cooled. The heat capacity of the calorimeter is the quantity of heat absorbed by the calorimeter for each 1C rise in temperature. We need to find the difference between the heat lost by the hot water when it droped from 600 to 400 and the heat gained by the cold water when it was heated up to 400 from 250.
DeltaH_diss -q_solution The minus sign is used here because heat lost carries a negative sign. More specifically you can assume that. A single burning match gives off approximately one BTU of heat.
View Answer Once the 100C water went to your stomach its. Two popular types of calorimeters are the coffee cup calorimeter and bomb calorimeter. This means that the reaction produces heat for the solution to absorb and q for the reaction is negative.
Calorimetry is the study of heat transfer and changes of state resulting from chemical reactions phase transitions or physical changes. Assume that all the heat released by the reaction was absorbed by the HCl solution and by the calorimeter. 1 g cm-3 Solution.
Shows molecules in two bodies at different temperatures and for hot and cold If two molecules collide energy transfers from the high-energy to the low-energy molecule. 42 J g-1 C-1. H ms T H 101 grams soln 4184 Jg C 645 C H -2720 J This is the amount of energy generated when 138 g of phosphorus trichloride is dissolved.
List the known quantities and plan the problem. Note that the values in the problem are in mL and the values in the solution are grams. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction.
How quickly heat flows through a material is called the materials U-Factor. And as well learn in another episode the extremely high specific heat capacity of water is due to the breaking in formation of hydrogen bonds that are associated with relatively small changes in temperature. Enthalpy of Solution Heat of Solution Example.
The tool used to measure heat change is the calorimeter. Express your answer in SI. The temperature change along with the specific heat and mass of the solution can then be used to calculate the amount of heat involved in either case.
In an experiment 12 g of sodium hydroxide pellets NaOH s were dissolved in 100 mL of water at 25C. The heat of fusion of ice is 333 Jg meaning 333 J is absorbed when 1 gram of ice melts The heat of vaporization of liquid water at 100C is 2257 Jg. Calculation of Molar Enthalpy heat of Solution 6.
The sensible heat formula with a change in temperature is expressed as. Calculate the heat energy that will be released when 50 kg of steam at 100C condenses to form water at 100C.

Thermal Capacity An Overview Sciencedirect Topics

Heat Absorbed During A Reaction Example Youtube

Solved Given The Following Information Below Calculate The Chegg Com
5 2 Calorimetry Chemistry

Eq How Can We Measure The Amount Of Heat Released Or Absorbed In A Chemical Reaction Do Now What Is The Difference Between Potential Energy And Kinetic Ppt Download

How To Calculate The Heat Gained By The Calorimeter
17 12 Multi Step Problems With Changes Of State Chemistry Libretexts
Calorimetry
