Nominal volumes of the volumetric glassware used in this exercise. Absorbance molar.
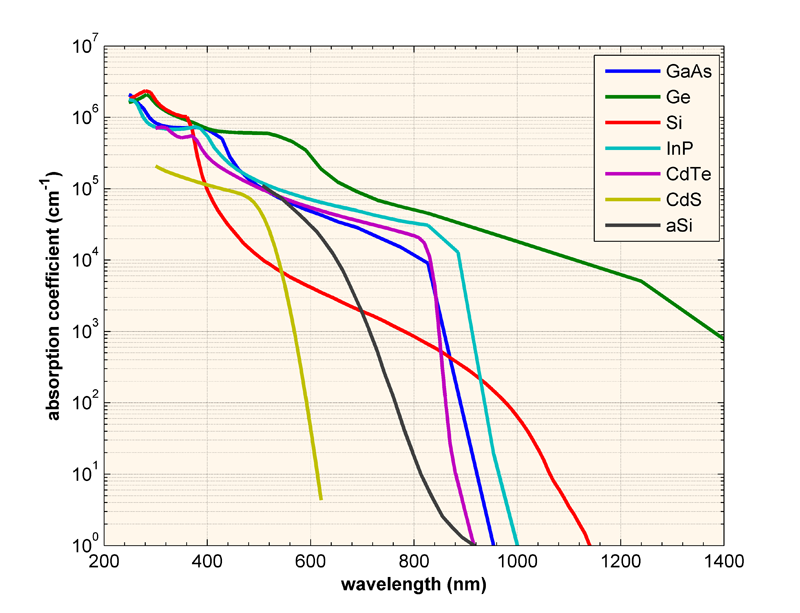
Absorption Coefficient Pveducation

And Figure 3 Also Show That As The Concentration Of Sample Increases Download Scientific Diagram
Nucleic Acid Quantitation Wikipedia
One can notice that at excitation wavelength of 532 nm a clear emission peak can be obtained approximately at 590 nm with the increase in signal intensity for each Rhodamine B concentration.
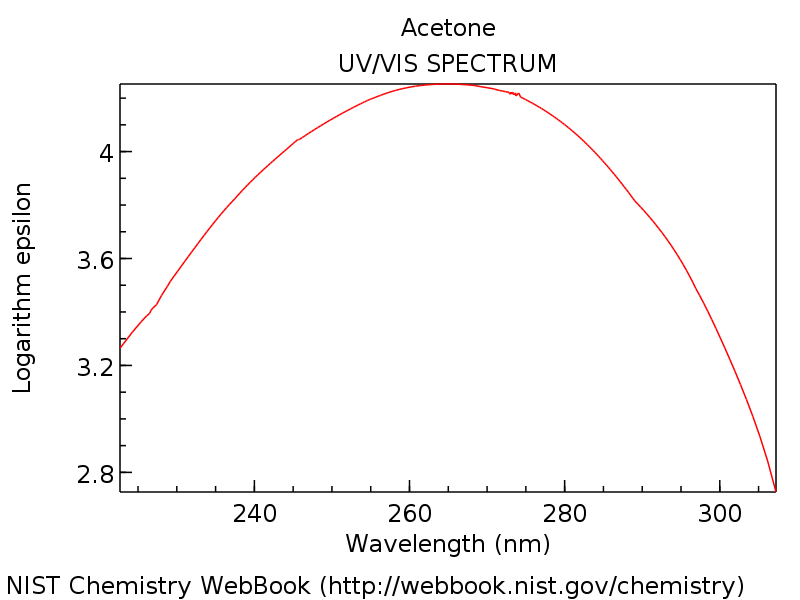
Why does absorbance increase with wavelength. For instance the absorbance of the alkene 2-methyl pent-2-ene is below 200 nm as is the ππ absorbance of 4-methyl pentane-2-one below. The linear relationship between concentration and absorbance is both simple and straightforward which is why we prefer to express the Beer-Lambert law using absorbance as a measure of the absorption rather than T. The signals travel farther which makes coverage easier and less costly.
The intensity of the absorption varies as a function of frequency and this variation is the absorption spectrum. What this also means is that the higher the molar absorptivity. Look at the images above.
Here is a modified diagram of the structure of the form in acidic solution - the colorless form. Absorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation as a function of frequency or wavelength due to its interaction with a sampleThe sample absorbs energy ie photons from the radiating field. Remember that the higher the molar absorptivity the higher the absorbance.
Does absorbance increase with wavelength. If any of these criteria do not apply there is the possibility to indirectly measure analytes andor using a. In general the absorbance depends on the wavelength.
Citation needed Unlike other protein assays the Bradford protein assay is less susceptible to interference by various chemical compounds such as sodium potassium or even carbohydrates like sucrose that may be present in protein samples. At lower frequencies a wavelength is longer making it more difficult to design antennas to fit into small devices. Infrared regions have so _ energy they usually just increase the movement of.
Figure 31 shows that absorbance increases with increased addition of carbon nanotubes because they play a role of radiation screener. Fortunately absorption and scattering of radiation by the flame are corrected by analyzing a blank. In mesityl oxide where the alkene and CO group are in conjugation with each other the absorption maximum moves to longer wavelength at 228 nm.
Absorbance of buffer reagents does not overlap with absorbance of the analyte. On a related note because transmittance has an inverse logarithmic relationship with absorbance this is why we cannot use transmittance in our calculations at least not. Indeed the entire vertical absorbance scale may be changed to a molar absorptivity scale once this information about the sample is in.
If the isoprene spectrum on the right was obtained from a dilute hexane solution c 4 10-5 moles per liter in a 1 cm sample cuvette a simple calculation using the above formula indicates a molar absorptivity of 20000 at the maximum absorption wavelength. The result is an apparent increase in the samples absorbance. Spectral interferences also occur when components of the samples matrix other than the analyte react to form molecular species such as oxides and hydroxides.
While at excitation wavelength of 570 nm there are no clear peaks but the signal increases with the concentration of the fluorescent dye. It should be noted that the absorbance of nanotubes at concentration of 008 wt the highest concentration on the graph was only about 10 times larger than absorbance of. The wavelength 410nm was a very good choice for the calibration plot but how do you know which wavelengt is the best wavelength based on the absorbance spectrum.
From the equation for the best-fit line for the absorbance at 525 nm as a function of Al 3 determine the percent aluminum by weight in alum and share your results with the class. The absorbance values we have adhere very closely to those predicted by the Beers law which states that absorbance is directly related to the concentration of a sample. The light at a specific wavelength Analyte wavelength is attenuated by the effect of flame components or matrix components in the sample Thus measured absorbance and analyte concentrations are too high If problem from flame blank aspiration will correct for it It is more serious at short wavelengths 430 nm and with.
This is done by first taking the pre-irradiated UV-vis absorbance of the sample and then using the equation below to calculate the SPF value. Cygnus Technologies EndonucleaseGTP ELISA Kit is a sensitive and specific assay to detect and quantify residual endonuclease impurity in recombinant viral vector and vaccine preparations. If we plot absorbance at the peak wavelength against concentration we get a straight line passing through the origin 00.
Js Speed of light c 2998 10. Where EE is the erythemal effect spectrum I is the solar intensity spectrum T is the sunscreen transmittance Abs is the sunscreen absorbance and CF 10 so that a standard 8 homosalate sunscreen would calculate to an SPF of 4. Measures hoe the wavelength of photons influences the amount of light absorbed by a pigment.
A microplate reader we use in our lab runs a linearity of. We can use a color wheel to predict the color we see based on the. Perform a Q-test on the class data and then calculate the average percent Al by weight in alum the standard deviation of the data and.
The increase of absorbance at 595 nm is proportional to the amount of bound dye and thus to the amount concentration of protein present in the sample. Using a standard curve. The absorbance A at a given wavelength is the logarithm to the base 10 of the ratio of the intensity of light having the given wavelength reaching the detector in the absence of the.
In this case the reflectivity and absorptivity of the body are interconnected. 10 from 0 to. When the transmitted light at 20Au is less than 1 an increase in concentration only changes absorbance a small amount.
However that also causes signals to interfere unless signals that cross into a common areaspace are differentiated in some fashion so that the interfering signals can be filtered by use of analog means or. Peaks indicate wavelength where absorbance or photosynthetic activity is high. Choosing Your Wavelength.
After the reaction has proceeded for several minutes does the amount of catalyst increase decrease or remain the same. One important consideration is the wavelength of radiation to use for the measurement. A blackbody does not exist and usually A 1 gray body.
Troughs where they are low. Expression of recombinant viral vectors and vaccines in mammalian cell culture is a cost-effective method for production of commercial quantities of gene therapy and vaccine biologics. Such substances show selective absorption see Fig.
So why does the color change as the structure changes. What we have is a shift to absorption at a higher wavelength in alkaline solution. As the number of double bonds increase the wavelength the molecule absorbs increases and the color that we see changes.
Wavelength Plancks constant h 6626 10 34. Absorbance or transmittance is then measured after 10 minutes but before 30 minutes using a color comparator with a scale in milligrams per liter that increases with the increase in color hue or an electronic meter that measures the amount of light absorbed or transmitted at a wavelength of 700 - 880 nanometers again depending on manufacturers directions. The calibration plot is like taking a vertical slice through the all the absorbance spectra at the specific wavelength 410nm.
As weve already seen a shift to higher wavelength is associated with a greater degree of delocalisation.

Uv Vis Spectroscopy Absorbance Of Carbonyls Master Organic Chemistry

User Elaine Marie Robbins Notebook Chem 496 2012 02 01 Openwetware

10 2 Spectroscopy Based On Absorption Chemistry Libretexts
Chem 125 Experiment Ii
![]()
Can Anyone Tell Me The Reason Why There Is Dip Decrease In Absorbance After Lamda Max In Uv Vis Spectra Of Semiconductor Nano Particles

Absorbance Vs Wavelength Graph Download Scientific Diagram

What Is The Effect Of The Wavelength On The Spectrophotometric Measurements Of Protein Biology Stack Exchange
3
