Absolute zero represents the coldest possible temperature which defies the this-versus-that pattern. Absolute temperature is temperature measured using the Kelvin scale where zero is absolute zero.
Facts About Absolute Zero Bright Hub Engineering
Absolute Zero And The Third Law Of Thermodynamics
Absolute Zero Temperature Nuclear Power
It was noted that gases seem to contract indefinitely as temperature.
What is absolute zero temperature. Absolute zero is equal to 27315 degrees Celsius. Temperature is an average of a part of the internal energy present in a body it does not include the energy of molecular binding or of molecular rotation. Its the lowest limit on the temperature scale but recent news articles have heralded a dip below that limit in a physics lab.
Gases are floating around you or trapped in bubbles. Solids are objects you can hold and maintain their shape. Absolute zero is the lowest possible temperature where nothing could be colder and no heat energy remains in a substance.
Stranger still absolute zero isnt even zero on the temperature scales used by nonscientists. Third law of thermodynamics states. Or 38 trillionths of a degree above absolute zero.
The temperature recorded in the lab reached 38 trillionths of a degree warmer than absolute zero reported DailyMail. Practically the work needed to remove heat from a gas increases the colder you. Absolute Zero and Third Law of Thermodynamics.
Matter at absolute zero has no remaining transferable average kinetic energy and the only remaining particle motion is due to an ever-pervasive quantum mechanical phenomenon called zero-point energy. This temperature is so low that its not even detectable with a regular thermometer of. Absolute zero is the point at which the fundamental particles of nature.
Gas Properties - PhET Interactive Simulations. Its minus 27315 degrees on the Celsius scale or minus 45967 degrees Fahrenheit or. The notion that there is an ultimately lowest temperature was suggested by the behaviour of gases at low pressures.
On the absolute temperature scale which is used by physicists and is also called the Kelvin scale it is not possible to go below zero at least not in the sense of getting colder than zero. This temperature is referred to as absolute zero and is the zero point for the Kelvin temperature scale. Examples of liquids at room temperature include water H 2 O blood and even honey.
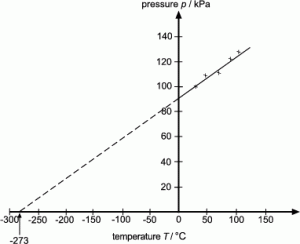
Absolute zerothe most appropriate temperature for both quantum experiments and quantum computingmakes it easier to describe a system by relying on a set of fundamental propositions. Liquids are found between the solid and gas states. The temperature at which the pressure of an ideal gas would in theory reach zero can be determined by extrapolating the pressure vs.
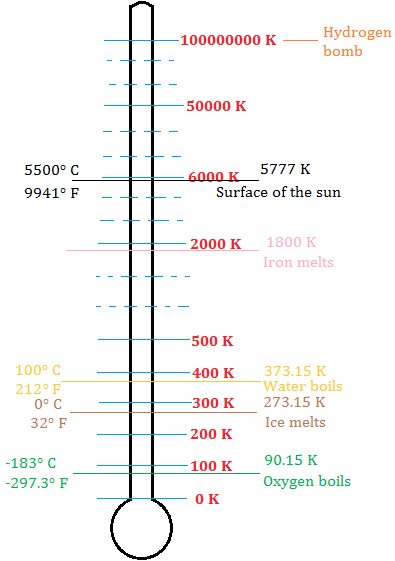
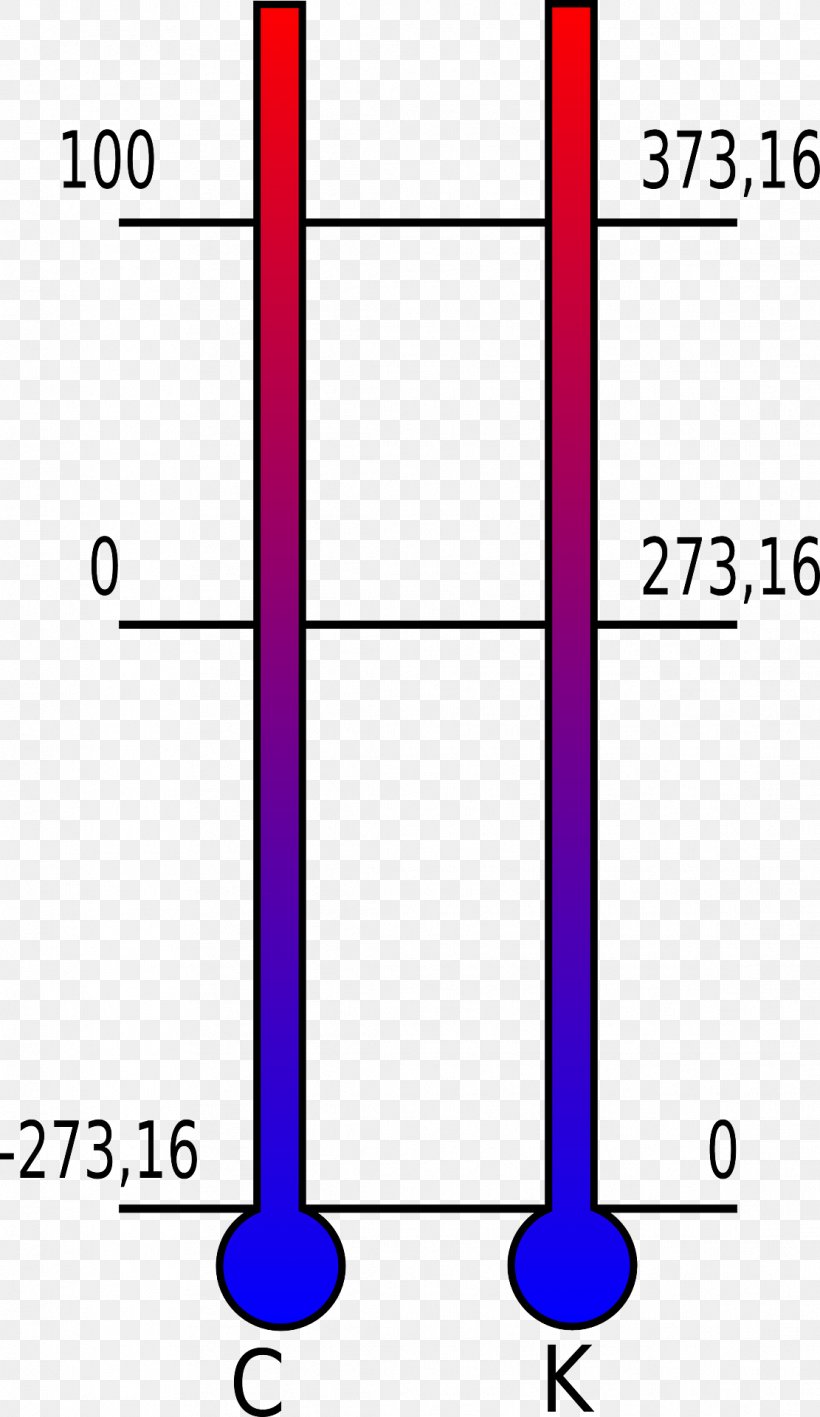
Temperature Scales and Absolute Zero Four Temperature Scales. It corresponds to 27315 C on the Celsius temperature scale and to 45967 F on the Fahrenheit temperature scale. The lowest possible energy state of a substance is defined as the absolute zero 27315 C or.
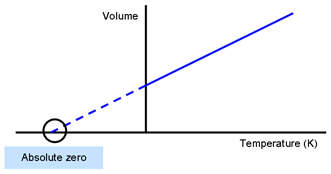
Carefully determine the temperature where your line crosses the X axis. While scientists have long suspected that theres an intrinsic speed limit on the act of cooling in our Universe that prevents us from ever achieving absolute zero 0 Kelvin -27315C or -45967F this is the strongest evidence yet that our current laws of physics hold true when it comes to the lowest possible temperature. If your value falls between -250 C and -300 you did well considering the limitations of your equipment and graph paper.
Absolute zero is the lowest limit of the thermodynamic temperature scale a state at which the enthalpy and entropy of a cooled ideal gas reach their minimum value taken as zero kelvinsThe fundamental particles of nature have minimum vibrational motion retaining only quantum mechanical zero-point energy-induced particle motionThe theoretical temperature is determined by extrapolating the. Why is absolute zero 0 kelvin or 27315C an impossible goal. This is obviously a far cry from more distant spaces 3 kelvins above absolute zero.
Absolute zero is the temperature at which a system is in the state of lowest possible minimum energy. Liquid Basics Liquids are the second state of matter we will talk about. The average temperature of outer space around the Earth is a balmy 28332 kelvins 1017 degrees Celsius or 503 degrees Fahrenheit.
This is your determination of absolute zero. Reportedly at present there are no thermometers to detect such a low temperature. Absolute zero is defined as the point where no more heat can be removed from a system according to the absolute or thermodynamic temperature scale.
The zero point is the temperature at which particles of matter have their minimum motion and can become no colder minimum energy. The most commonly used temperature scale in the US today is the Fahrenheit scale abbreviated F. Absolute temperature also called thermodynamic temperature is the temperature of an object on a scale where 0 is taken as absolute zeroAbsolute temperature scales are Kelvin and Rankine.
If you have different types of molecules dissolved in a liquid it is. Absolute zero temperature at which a thermodynamic system has the lowest energy. This only holds strictly when atmospheric pressure equals the average sea level pressure.
Attach the pressure gauge on the Boyles law apparatus to a metal sphere. Temperature graph to zero pressure. The Nernst-Simon statement of the 3 rd law of thermodynamics can be written as.
For a condensed system undergoing an isothermal process that is reversible in nature the associated entropy change approaches zero as the associated temperature approaches zero. Absolute zero which is a temperature of zero kelvins 0 K is precisely equal to 27315 C and 45967 F. The entropy of a system approaches a constant value as the temperature approaches absolute zero.
Absolute zero is denoted as 0 K on the Kelvin scale 27315 C on the Celsius scale and 45967 F on the Fahrenheit scale. Immerse the sphere in one of the different temperature baths. Hot warm room temp ice and slush.
The expected value for absolute zero is -27315 C. Absolute zero or 0 degrees Kelvin is the temperature where all motion stops. However the entropy at absolute zero can be equal to zero as is the case when a perfect crystal is considered.
And record the temperature of the bath and pressure of the gas within the sphere. Absolute zero pressuretemperature relationship in gases Demonstration. The researchers from the University of Bremen were able to create one of the coldest places in the universe for a few seconds in the lab.
We show that you cant actually cool a system to absolute zero. Because it is absolute a thermodynamic temperature reading is not followed by a degree symbol. In this scale water freezes at 32 degrees and boils at 212 degrees.
This corresponds to zero Kelvin or minus 27315 CThis is zero on the Rankine scale and minus 45967 F.
Kelvin Celsius Thermometer Degree Absolute Zero Png 1111x1920px Kelvin Absolute Zero Area Celsius Color Temperature Download
What Temperature Scale Starts At Absolute Zero Socratic
5 16 Understand Why There Is An Absolute Zero Of Temperature Which Is 273 C Tutormyself Chemistry

Scientists Say Absolute Zero Science News For Students
Atoms Reach Record Temperature Colder Than Absolute Zero Live Science

Solved Absolute Zero Temperature 1 Define Temperature The Chegg Com
Episode 602 Ideal Gases And Absolute Zero Iopspark

The Journey To The Other Side Of Absolute Zero

